IDEATION – Build Compliant Products
from the Start
Centralize all Project Info
Avoid missing changes made to marketing briefs and keep your product development team on the same page.
Fast Track Development
Find ingredients to use in your formulations and review regulatory and safety data on any ingredient. Check formulas against Retailer Clean Lists.
Step-by-Step Guidance
Follow our guided process to qualify and approve new Raw Materials for your formulations.
Find Ingredients, Build Compliant Products, Access Documents Easily
The IIN is the only ingredient, regulatory, and document management software, which enables you to create compliant products from the start.
Build compliant products from the start
Using Formul8, companies can track the status of projects, gather assets, distribute documents and record due dates all in one central location. Every member of the project team can access important documents quickly and easily, making it easier to meet deadlines and reduce costs along the way.
The IIN offers a new way for companies to manage their marketing briefs and project information while capturing all relevant information needed for product development.

Quickly find ingredients to use or replace in a formula
Search ingredients using the IIN and find information on concentration, restrictions and usage levels. Ingredient Search from a database of ingredients to use in your formulations and review regulatory and safety data on any ingredient.

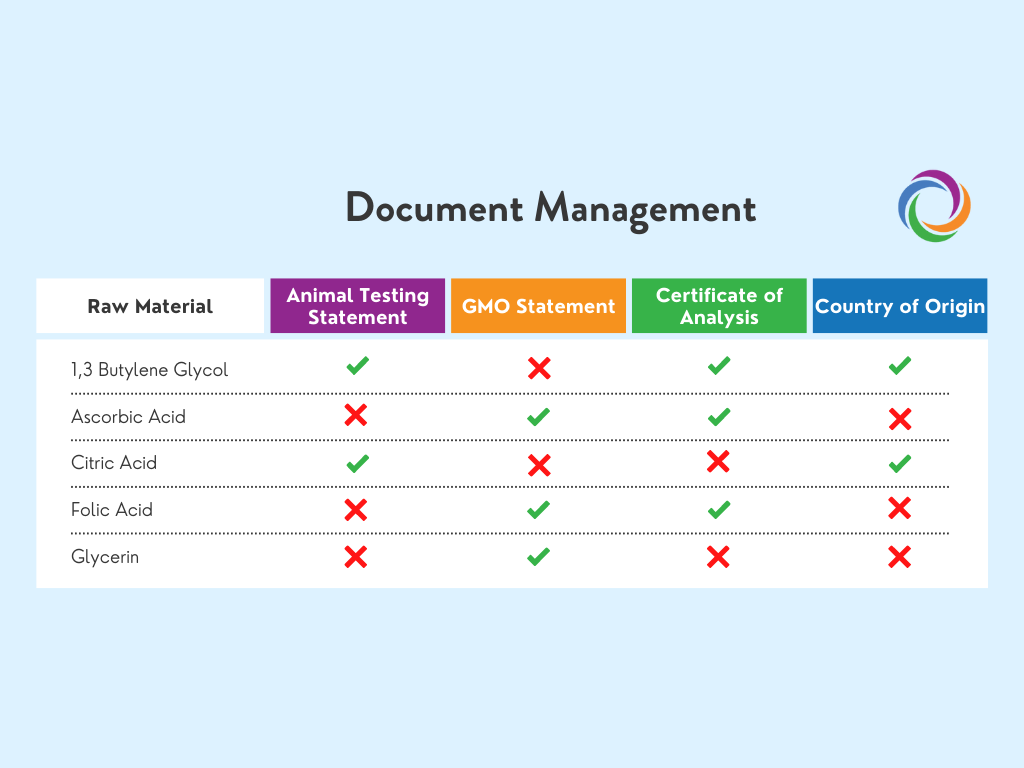
Access all raw material documentation in one place
Qualify/Validate ingredients before you use them. Set standard requirements for raw materials before you use them. Make sure everyone who needs access to documentation, has it, and put controls in place to maintain the quality of your information.
What Stage are you at in New Product Development?
NPD Wheel
Click around the spokes of the Ithos NPD Wheel to learn more about each stage!
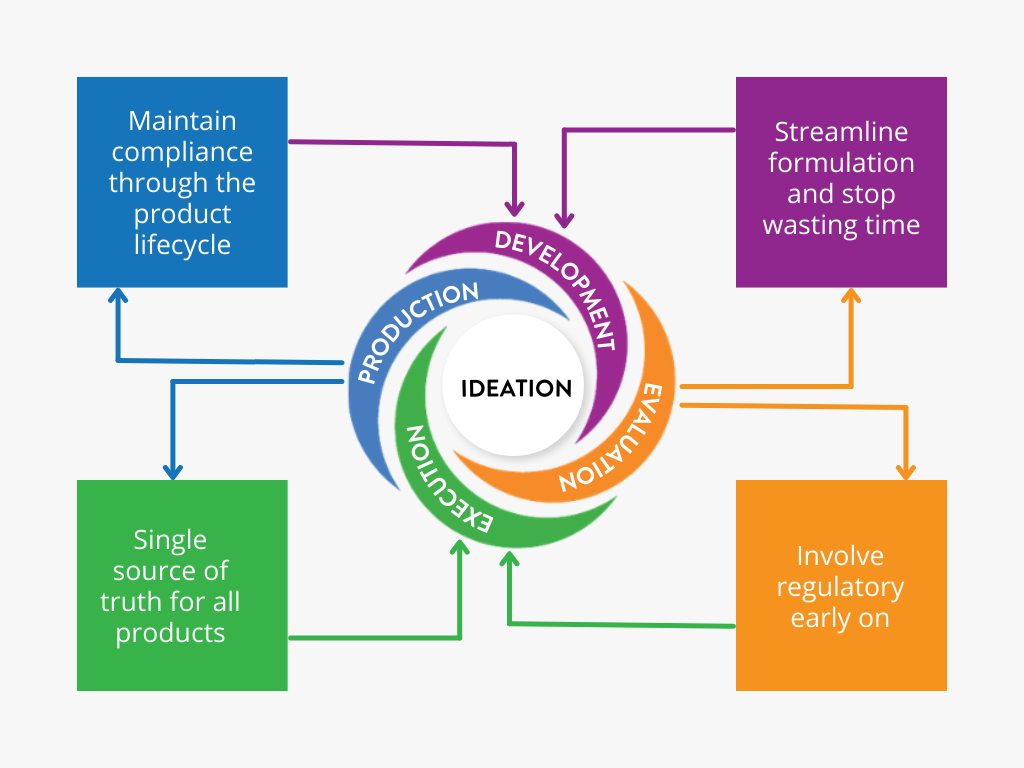
Ideation
Is it difficult to get everyone on the same page for new product development?
Do you receive incomplete marketing briefs? Or miss changes made to it after the fact?
Development
Do you have trouble tracking the various tests for formulas or knowing what testing is needed?
How do you track the reasons for reformulation?
Need to find alternative ingredients?
Evaluation
Do you have trouble gathering missing documents for your RMs?
Does it take you too long to review formulas for global compliance?
Is it hard for you to stay on top of the changing ingredient laws?
Execution
Are you missing documents needed for product registrations?
Does it take a lot of time for you to gather missing documents?
Do you need SDSs quickly, last minute?
Production
Do you find yourself facing the costs associated with noncompliance due to an ingredient ban or usage restriction?
Have you experienced difficulty maintaining compliance with changing regulations that require additional reporting?
Is it hard to find where an ingredient is used across your product portfolio?
Do you often miss expired docs until you need them?
Is it difficult to track finished good imports and the required data points?
Development
Do you have trouble tracking the various tests for formulas or knowing what testing is needed?
How do you track the reasons for reformulation?
Need to find alternative ingredients?
Evaluation
Do you have trouble gathering missing documents for your RMs?
Does it take you too long to review formulas for global compliance?
Is it hard for you to stay on top of the changing ingredient laws?
Execution
Are you missing documents needed for product registrations?
Does it take a lot of time for you to gather missing documents?
Do you need SDSs quickly, last minute?
Production
Do you find yourself facing the costs associated with noncompliance due to an ingredient ban or usage restriction?
Have you experienced difficulty maintaining compliance with changing regulations that require additional reporting?
Is it hard to find where an ingredient is used across your product portfolio?
Do you often miss expired docs until you need them?
Is it difficult to track finished good imports and the required data points?
Trusted by Leading Cosmetics Organizations
Subscribe to Our Newsletter
Sign up to receive updates from Ithos Global, including information about upcoming events, regulatory news, and more.
Ithos Global Names John Bender as New President to Lead Growth and Expansion
Last Updated on July 11, 2024 by Ithos Global Regulatory Team Experienced SaaS Industry Executive to Drive Growth and Operational…
3 Reasons to Come See Us at NYSCC Suppliers’ Day 2024!
Last Updated on April 16, 2024 by Ithos Global Regulatory Team We‘re heading back to the Big Apple May 1-2…
Debunking MoCRA Myths
MoCRA Myths Debunked: Reporting Tips to Save You Time Like anything new, there’s some misinformation floating around about MoCRA. And…
8 Things You Can Do Now to Prepare for MoCRA Webinar
SUMMARY The Modernization of Cosmetics Regulation Act (MoCRA) was signed into law as part of the 2023 Omnibus Bill. It…
FDA
US Food and Drug Administration (a US federal regulator)
GHS
“Globally Harmonized System for Classification and Labeling of Chemicals” – 2003 UN protocol for standardization of information.
RP
“Responsible Person” Responsible Person is a legal or natural person based in the European Union who will act as the…
Wednesday, May 29th ‘Debunking MoCRA Myths’ Webinar Signup
MoCRA Myths Debunked: Reporting Tips to Save You Time Wednesday, May 29th 12pm – 1pm EST Since the Modernization…
May 2024 MoCRA Webinar Signup
Join us for two free webinars! Our regulatory team is guiding brands, manufacturers, retailers and labs on MoCRA compliance. Now,…