PRODUCTION- Maintain Compliance
through the Product Lifecycle
Easily Find Ingredients
Identify and track the location of all your ingredients, ingredient attributes, substances and documents for each product using the IIN’s ‘Where Used’ feature.
Eliminate Manual Processes
Save time and money by integrating regulatory content and import tracking directly with your Product Life-cycle Management (PLM) system.
Receive Regulatory Updates
Stay up-to-date with regulatory changes and get notified immediately if any of your products are impacted by changing regulations.
Find Ingredients, Build Compliant Products, Access Documents Easily
The IIN is the only ingredient, regulatory, and document management software, which enables you to create compliant products from the start.
Quickly share documentation
When it comes to keeping track of product documentation, it’s no longer a matter of who, what, where and when. The IIN includes custom confidentiality settings, so only relevant parties have access to your intellectual property. Document status history is automatically captured and made available to you in real time. See when document requests were made, when they were received, who made modifications and when they were made.
Stay on top of compliance after product launch
With the IIN’s regulatory surveillance function, you know up front if any of your products are impacted by changing regulations. In addition to quarterly updated reports, you’ll be able to monitor your products’ compliance history, manage documents and track product information and changes through all stages of the product life cycle.
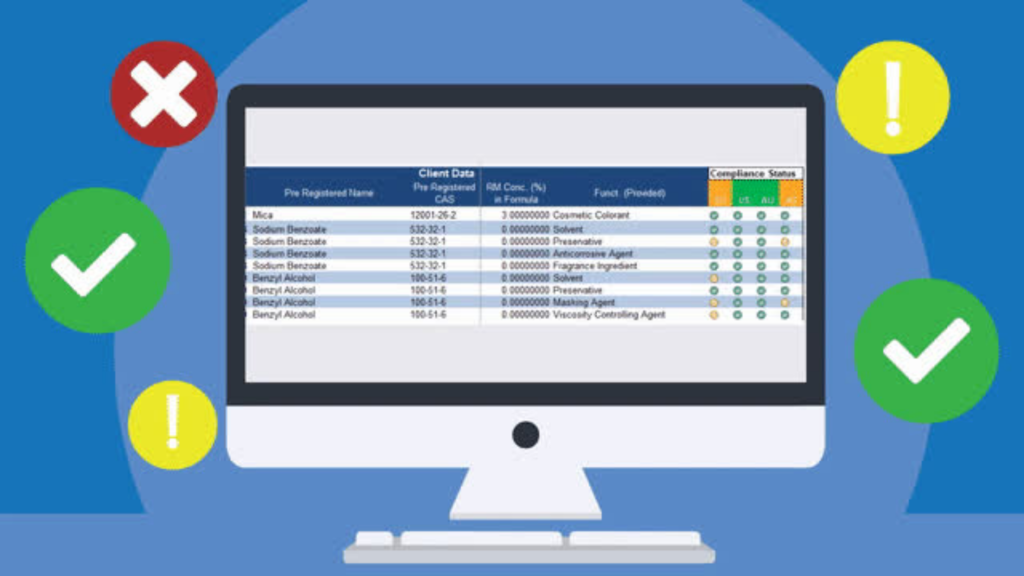
Quickly find where ingredients are used across entire product portfolio
It’s tough to know where an ingredient, ingredient attribute or substance is located in your product portfolio, especially if you have a large number of products. The IIN’s Where Used search feature allows you to run your own search filters with criteria such as ingredient name, Raw Material, substance or raw material supplier. Search to view which formulas contain certain substances or raw materials or where a substance appears in a formula or raw material. All search results can be categorized and exported into a spreadsheet for easy visibility.

What Stage are you at in New Product Development?
NPD Wheel
Click around the spokes of the Ithos NPD Wheel to learn more about each stage!
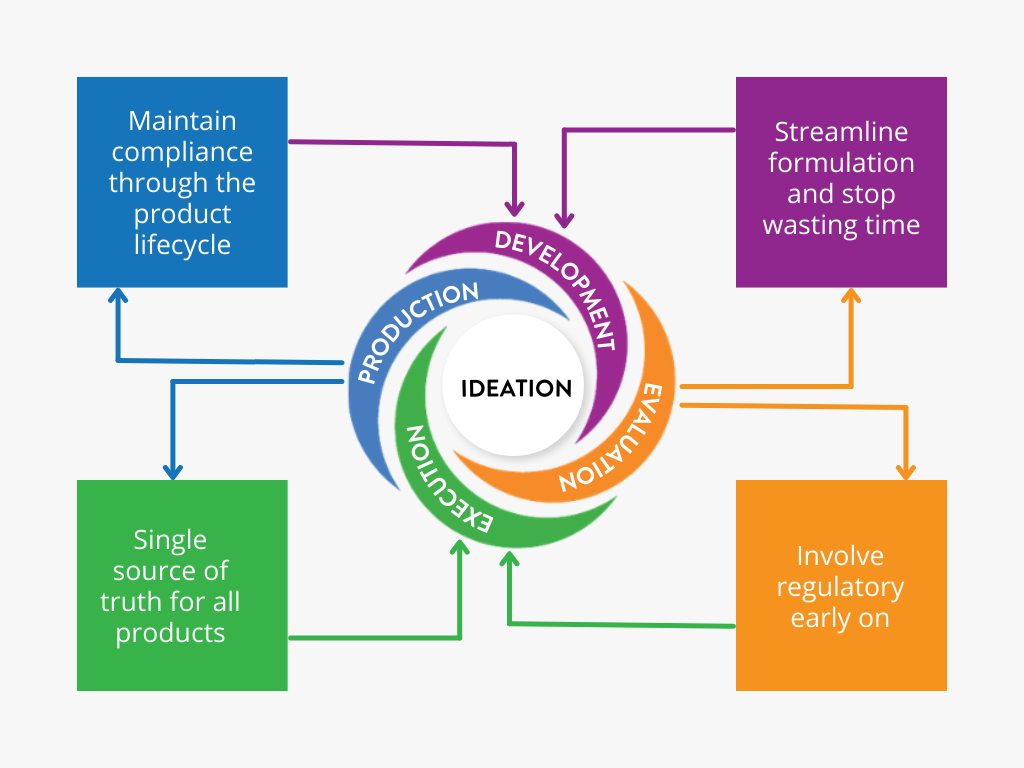
Ideation
Is it difficult to get everyone on the same page for new product development?
Do you receive incomplete marketing briefs? Or miss changes made to it after the fact?
Development
Do you have trouble tracking the various tests for formulas or knowing what testing is needed?
How do you track the reasons for reformulation?
Need to find alternative ingredients?
Evaluation
Do you have trouble gathering missing documents for your RMs?
Does it take you too long to review formulas for global compliance?
Is it hard for you to stay on top of the changing ingredient laws?
Execution
Are you missing documents needed for product registrations?
Does it take a lot of time for you to gather missing documents?
Do you need SDSs quickly, last minute?
Production
Do you find yourself facing the costs associated with noncompliance due to an ingredient ban or usage restriction?
Have you experienced difficulty maintaining compliance with changing regulations that require additional reporting?
Is it hard to find where an ingredient is used across your product portfolio?
Do you often miss expired docs until you need them?
Is it difficult to track finished good imports and the required data points?
Development
Do you have trouble tracking the various tests for formulas or knowing what testing is needed?
How do you track the reasons for reformulation?
Need to find alternative ingredients?
Evaluation
Do you have trouble gathering missing documents for your RMs?
Does it take you too long to review formulas for global compliance?
Is it hard for you to stay on top of the changing ingredient laws?
Execution
Are you missing documents needed for product registrations?
Does it take a lot of time for you to gather missing documents?
Do you need SDSs quickly, last minute?
Production
Do you find yourself facing the costs associated with noncompliance due to an ingredient ban or usage restriction?
Have you experienced difficulty maintaining compliance with changing regulations that require additional reporting?
Is it hard to find where an ingredient is used across your product portfolio?
Do you often miss expired docs until you need them?
Is it difficult to track finished good imports and the required data points?
Trusted by Leading Cosmetics Organizations
Subscribe to Our Newsletter
Sign up to receive updates from Ithos Global, including information about upcoming events, regulatory news, and more.
Ithos Global Names John Bender as New President to Lead Growth and Expansion
Last Updated on July 11, 2024 by Ithos Global Regulatory Team Experienced SaaS Industry Executive to Drive Growth and Operational…
3 Reasons to Come See Us at NYSCC Suppliers’ Day 2024!
Last Updated on April 16, 2024 by Ithos Global Regulatory Team We‘re heading back to the Big Apple May 1-2…
Debunking MoCRA Myths
MoCRA Myths Debunked: Reporting Tips to Save You Time Like anything new, there’s some misinformation floating around about MoCRA. And…
8 Things You Can Do Now to Prepare for MoCRA Webinar
SUMMARY The Modernization of Cosmetics Regulation Act (MoCRA) was signed into law as part of the 2023 Omnibus Bill. It…
PIF
“Product Information Form” industry or jurisdiction-agreed standard format for capturing key product details (e.g. PIFs per EU regulation 1223/2009)
Dual INCI’s
US vs EU naming conventions for INCI substances, e.g. “water” vs. “aqua”
GHS
“Globally Harmonized System for Classification and Labeling of Chemicals” – 2003 UN protocol for standardization of information.
Wednesday, May 29th ‘Debunking MoCRA Myths’ Webinar Signup
MoCRA Myths Debunked: Reporting Tips to Save You Time Wednesday, May 29th 12pm – 1pm EST Since the Modernization…
May 2024 MoCRA Webinar Signup
Join us for two free webinars! Our regulatory team is guiding brands, manufacturers, retailers and labs on MoCRA compliance. Now,…